Intro video
In both prokaryotes and eukaryotes, the second function of DNA (the first was replication) is to provide the information needed to construct the proteins necessary so that the cell can perform all of its functions. To do this, the DNA is “read” or transcribed into an mRNA molecule. The mRNA then provides the code to form a protein by a process called translation. Through the processes of transcription and translation, a protein is built with a specific sequence of amino acids that was originally encoded in the DNA. This module discusses the details of transcription.
The Central Dogma: DNA Encodes RNA; RNA Encodes Protein
The flow of genetic information in cells from DNA to mRNA to protein is described by the central dogma which states that genes specify the sequences of mRNAs, which in turn specify the sequences of proteins.
The central dogma states that DNA encodes RNA, which in turn encodes protein.
The copying of DNA to mRNA is relatively straightforward, with one nucleotide being added to the mRNA strand for every complementary nucleotide read in the DNA strand. The translation to protein is more complex because groups of three mRNA nucleotides correspond to one amino acid of the protein sequence. However, as we shall see in the next module, the translation to protein is still systematic, such that nucleotides 1 to 3 correspond to amino acid 1, nucleotides 4 to 6 correspond to amino acid 2, and so on.
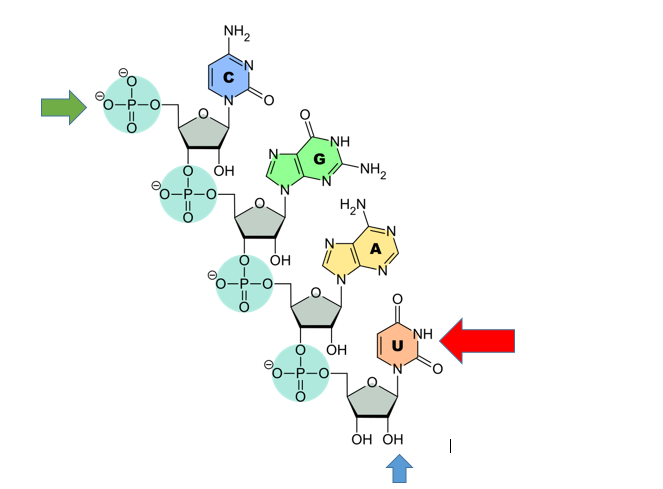
RNA features
RNA contains U, not T. (Red arrow). U pairs with A
RNA contains ribose. The Blue arrow points to the ribose sugar. Note the oxygen. DNA would just have a hydrogen there. The hydroxyl group to the left is the 3’ hydroxyl (like DNA the Green arrow points to the 5’ phosphate.
Note that this RNA is single stranded. RNA usually is single stranded unlike DNA. Sometimes RNA will pair with itself.
Transcription: from DNA to mRNA
Both prokaryotes and eukaryotes perform fundamentally the same process of transcription, with the important difference of the membrane-bound nucleus in eukaryotes. With the genes bound in the nucleus, transcription occurs in the nucleus of the cell and the mRNA transcript must be transported to the cytoplasm. The prokaryotes, which include bacteria and archaea, lack membrane-bound nuclei and other organelles, and transcription occurs in the cytoplasm of the cell. In both prokaryotes and eukaryotes, transcription occurs in three main stages: initiation, elongation, and termination.
Initiation
Transcription requires the DNA double helix to partially unwind in the region of mRNA synthesis. The region of unwinding is called a transcription bubble. The DNA sequence onto which the proteins and enzymes involved in transcription bind to initiate the process is called a promoter. In most cases, promoters exist upstream of the genes they regulate. The specific sequence of a promoter is very important because it determines whether the corresponding gene is transcribed all of the time, some of the time, or hardly at all (Figure).
The initiation of transcription begins when DNA is unwound, forming a transcription bubble. Enzymes and other proteins involved in transcription bind at the promoter.
Elongation
Transcription always proceeds from one of the two DNA strands, which is called the template strand. The mRNA product is complementary to the template strand and is almost identical to the other DNA strand, called the nontemplate strand, with the exception that RNA contains a uracil (U) in place of the thymine (T) found in DNA. During elongation, an enzyme called RNA polymerase proceeds along the DNA template adding nucleotides by base pairing with the DNA template in a manner similar to DNA replication, with the difference that an RNA strand is being synthesized that does not remain bound to the DNA template. As elongation proceeds, the DNA is continuously unwound ahead of the core enzyme and rewound behind it (Figure).
During elongation, RNA polymerase tracks along the DNA template, synthesizes mRNA in the 5′ to 3′ direction, and unwinds then rewinds the DNA as it is read.
Problem example
A DNA is 3′ TAC AGC TTG 5′ What is the RNA that can be made from it?
5′ AUG UCG AAC 3′
3′ TAC AGC TTG 5′
Note that C and G pairing is just like DNA. U in RNA pairs with A in DNA. T in DNA pairs with A in RNA like DNA.
Transcription video
Splicing and other RNA processing
Eukaryotic RNA Processing
The newly transcribed eukaryotic mRNAs must undergo several processing steps before they can be transferred from the nucleus to the cytoplasm and translated into a protein. The additional steps involved in eukaryotic mRNA maturation create a molecule that is much more stable than a prokaryotic mRNA. For example, eukaryotic mRNAs last for several hours, whereas the typical prokaryotic mRNA lasts no more than five seconds.
The mRNA transcript is first coated in RNA-stabilizing proteins to prevent it from degrading while it is processed and exported out of the nucleus. This occurs while the pre-mRNA still is being synthesized by adding a special nucleotide “cap” to the 5′ end of the growing transcript. In addition to preventing degradation, factors involved in protein synthesis recognize the cap to help initiate translation by ribosomes.
Once elongation is complete, an enzyme then adds a string of approximately 200 adenine residues to the 3′ end, called the poly-A tail. This modification further protects the pre-mRNA from degradation and signals to cellular factors that the transcript needs to be exported to the cytoplasm.
Eukaryotic genes are composed of protein-coding sequences called exons (ex-on signifies that they are expressed) and intervening sequences called introns (int-ron denotes their intervening role). Introns are removed from the pre-mRNA during processing. Intron sequences in mRNA do not encode functional proteins. It is essential that all of a pre-mRNA’s introns be completely and precisely removed before protein synthesis so that the exons join together to code for the correct amino acids. If the process errs by even a single nucleotide, the sequence of the rejoined exons would be shifted, and the resulting protein would be nonfunctional. The process of removing introns and reconnecting exons is called splicing (Figure). Introns are removed and degraded while the pre-mRNA is still in the nucleus.

Eukaryotic mRNA contains introns that must be spliced out. A 5′ cap and 3′ tail are also added.
Note that the RNA contained 3 exons and 2 introns. RNAs begin and end with exons. There are always 1 more exon than intron in the primary transcript.
The video below shows the relationship between
Why do this? What is the point of removing introns? They are not used, they are just degraded once removed. One reason is alternative splicing. IN this process, some exons cam be removed during splicing. This can yield more than one protein from the same RNA. The following lesson explains alternate splicing
Translation
The synthesis of proteins is one of a cell’s most energy-consuming metabolic processes. In turn, proteins account for more mass than any other component of living organisms (with the exception of water), and proteins perform a wide variety of the functions of a cell. The process of translation, or protein synthesis, involves decoding an mRNA message into a polypeptide product. Amino acids are covalently strung together in lengths ranging from approximately 50 amino acids to more than 1,000.
The Protein Synthesis Machinery
In addition to the mRNA template, many other molecules contribute to the process of translation. The composition of each component may vary across species; for instance, ribosomes may consist of different numbers of ribosomal RNAs (rRNA) and polypeptides depending on the organism. However, the general structures and functions of the protein synthesis machinery are comparable from bacteria to human cells. Translation requires the input of an mRNA template, ribosomes, tRNAs, and various enzymatic factors (Figure 9.19).
In E. coli, there are 200,000 ribosomes present in every cell at any given time. A ribosome is a complex macromolecule composed of structural and catalytic rRNAs, and many distinct polypeptides. In eukaryotes, the nucleolus is completely specialized for the synthesis and assembly of rRNAs.
Ribosomes are located in the cytoplasm in prokaryotes and in the cytoplasm and endoplasmic reticulum of eukaryotes. Ribosomes are made up of a large and a small subunit that come together for translation. The small subunit is responsible for binding the mRNA template, whereas the large subunit sequentially binds tRNAs, a type of RNA molecule that brings amino acids to the growing chain of the polypeptide. Each mRNA molecule is simultaneously translated by many ribosomes, all synthesizing protein in the same direction.
Depending on the species, 40 to 60 types of tRNA exist in the cytoplasm. Serving as adaptors, specific tRNAs bind to sequences on the mRNA template and add the corresponding amino acid to the polypeptide chain. Therefore, tRNAs are the molecules that actually “translate” the language of RNA into the language of proteins. For each tRNA to function, it must have its specific amino acid bonded to it. In the process of tRNA “charging,” each tRNA molecule is bonded to its correct amino acid.
The Genetic Code
To summarize what we know to this point, the cellular process of transcription generates messenger RNA (mRNA), a mobile molecular copy of one or more genes with an alphabet of A, C, G, and uracil (U). Translation of the mRNA template converts nucleotide-based genetic information into a protein product. Protein sequences consist of 20 commonly occurring amino acids; therefore, it can be said that the protein alphabet consists of 20 letters. Each amino acid is defined by a three-nucleotide sequence called the triplet codon. The relationship between a nucleotide codon and its corresponding amino acid is called the genetic code.
Given the different numbers of “letters” in the mRNA and protein “alphabets,” combinations of nucleotides corresponded to single amino acids. Using a three-nucleotide code means that there are a total of 64 (4 × 4 × 4) possible combinations; therefore, a given amino acid is encoded by more than one nucleotide triplet (Figure 9.20).
Three of the 64 codons terminate protein synthesis and release the polypeptide from the translation machinery. These triplets are called stop codons. Another codon, AUG, also has a special function. In addition to specifying the amino acid methionine, it also serves as the start codon to initiate translation. The reading frame for translation is set by the AUG start codon near the 5′ end of the mRNA. The genetic code is universal. With a few exceptions, virtually all species use the same genetic code for protein synthesis, which is powerful evidence that all life on Earth shares a common origin.
Problem
Problem example
A DNA is 3′ TAC AGC TTG 5′ What is the Protein that can be made from it?
5′ AUG UCG AAC 3′ is the RNA
MET CYS ASN is the amino acid sequence
You will not have to memorize the codon table
You will have to be able to use it
The Mechanism of Protein Synthesis
Just as with mRNA synthesis, protein synthesis can be divided into three phases: initiation, elongation, and termination. The process of translation is similar in prokaryotes and eukaryotes. Here we will explore how translation occurs in E. coli, a representative prokaryote, and specify any differences between prokaryotic and eukaryotic translation.
Protein synthesis begins with the formation of an initiation complex. In E. coli, this complex involves the small ribosome subunit, the mRNA template, three initiation factors, and a special initiator tRNA. The initiator tRNA interacts with the AUG start codon, and links to a special form of the amino acid methionine that is typically removed from the polypeptide after translation is complete.
In prokaryotes and eukaryotes, the basics of polypeptide elongation are the same, so we will review elongation from the perspective of E. coli. The large ribosomal subunit of E. coli consists of three compartments: the A site binds incoming charged tRNAs (tRNAs with their attached specific amino acids). The P site binds charged tRNAs carrying amino acids that have formed bonds with the growing polypeptide chain but have not yet dissociated from their corresponding tRNA. The E site releases dissociated tRNAs so they can be recharged with free amino acids. The ribosome shifts one codon at a time, catalyzing each process that occurs in the three sites. With each step, a charged tRNA enters the complex, the polypeptide becomes one amino acid longer, and an uncharged tRNA departs. The energy for each bond between amino acids is derived from GTP, a molecule similar to ATP (Figure 9.21). Amazingly, the E. coli translation apparatus takes only 0.05 seconds to add each amino acid, meaning that a 200-amino acid polypeptide could be translated in just 10 seconds.