Be able to list and recognize the principles of the cell theory
This video is useful and fun.
Be able to give applications of GFP
. In order to examine moving structures in living cells scientists took advantage of a jelly fish which makes a protein which glows green in fluorescent light. This protein is called GFP.(Green fluorescence protein) Using molecular tricks, one can fuse the GFP protein to other proteins. Where ever that other protein is, there will be a green signal. This video shows an example:
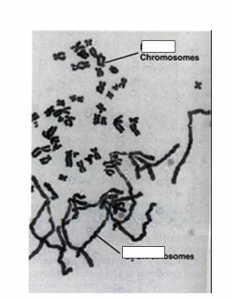
In this video, a protein associated with chromosomes is labeled green. First, you can see the nucleii from the 2 parents fuse together. Then the embryo starts cell division and you can follow the nucleii as this happens. The organism is C. elegans which is a tiny worm.
Cell Parts. Here are some activities to help you remember cell parts and functions
Here is a review video
(Click on the flag and then, when prompted, click on the red dot associated with the structure)
Two structures that easily get confused are the nucleus and the nucleolus
Note the difference.
The function of the nucleolus is to help make ribosomes
The nucleus stores the chromosomes which have the genetic information for a cell. The nucleus is also needed for cell division and contains the nucleolus.
Know the pathway that membrane and secreted proteins take to get to or through the cell membrane. Know what happens at each step.
Know the functions of cytoskeletal proteins including microtubules microfilaments and intermediate filaments. Be able to give examples of how these elements contribute to movement of cells and of structures within cells and how they are involved in structure and shape of cells.
here is a video on cilia function
Be able to give examples of how human disorders relate to defects in specific cell structures and/or proteins.
Here is a video on lysosomal storage disease
This boy has a condition called EBS (Epidermolysis Bullosa Simplex), which causes severe blistering. This is a genetic condition which can be caused by defects in a number of proteins including keratin (an intermediate filament protein in the skin) and in components of the anchoring junctions. Based on what you have read about the functions of these components, why might a defect in them results in the blistering condition?
https://commons.wikimedia.org/w/index.php?curid=9793806
These are red blood cells from someone who has a defect called Hereditary Spherocytosis. Normal red blood cells are disc shaped. In the microscope, they will usually shown a clearer area in the center. Abnormal red blood cells are rounder and do not have the clear area in the center. Depending on the severity of the disease, the patient will have different numbers of normal and rounder red blood cells. The condition is genetic and is due to defects in a number of proteins which are known to associate with actin. Based on what you know about actin, explain the effect of the defect on the cells.
Extracellular Matrix; In addition to what is in the book, you may find this video quite informative
Here is a useful review activity
Use the “match’ Function to do a speed test.